Note – Yes, I know I published this well into my sophomore year. I wrote this in the summer.
I don’t think I’ve put more effort into anything else.
Author’s note right before publishing: No, I cooked.
I have to do AP Chem next year. I’m screwed. (In my sophomore year) (Me in my sophomore year: HELP. THIS COURSE WAS NOT DIFFICULT AT ALL COMPARED TO THE AP)
Aaaaactually, maybe I’m doing something wrong myself. The class frankly wasn’t that hard for most people, and the A-rate for it is surprisingly high, despite just about everyone talking on and on about how it’s their hardest class or how it’s really difficult. I got an A in it just like the rest, but for a while it looked like that wasn’t going to happen and I would bet money that I put more effort into that class than anyone else.
I’m not joking- I fought with my parents so much over work ethic when it came to this class, and it’s not because I wasn’t working hard enough. It’s because everyone thought I was working too hard.
We’ll start by going over the curriculum.
Unit 5 – Reactions, Kinetics, Stoichiometry, Equilibrium, and Collision theory: This one probably the largest unit of the whole trimester. They talked about pretty much everything related to reactions; they started with chemical vs physical reactions (the difference is one changes chemical structure and physical reactions don’t), as well as the types of reactions (Which we went over in pretty much every class). They can be distinguished pretty much solely on their chemical equation, and there are 5 types, as noted here:
Synthesis – A+B→ AB
Decomposition – AB→A+B (So basically Synthesis’s opposite)
Single Replacement – AB+C→ AC+B
Double Replacement – AB+CD→ AC+BD
Combustion – A+O2-> other stuff (kinda different, just make sure it balances out)
These aren’t too difficult to identify on their own, but this is only the beginning of the crazy amount of material that they’d throw at us. Each equation needs to also be balanced- in other words, the reactants has to have the same number of every element as the products. This is a fairly easy concept, just visualize out all the elements in one way or another, and you should be able to multiply things and balance it out, it simply takes time.
And then we got to one of the most important concepts in all of chemistry… the mole. And no, I’m not going to make a joke about the mole animal because that’s already happened too many times.
The mole is, in short, a unit which allows for a comparison between the mass of something and a number of particles. Every element and compound has this thing called a molar mass, which is unique to them. It’s typically used in calculations, and the first application is stoichiometry.
Stoichiometry is essentially just unit conversions through chemical equations. With this, you can figure out how much product you get in a reaction given the amount that goes out. There’s a lot of further applications to stoich that they talk about in later units, but for now we’re just doing reactions.
There’s also this thing called Collision Theory, which states that particles have to go through this thing called an “effective collision” in order to actually have a reaction. The number of collisions that are effective that you have increases the speed in which a reaction occurs. There are a few factors that influence reaction rate, including temperature, pressure, and catalyst. A catalyst is essentially a material you add to a reaction to speed it up, and it does that by lowering the energy needed to undergo an effective collision.
They also wanted us to understand how the graphs in the energy of a reaction work, known as an “Energy Profile”. The slope you see comes from the activation energy, and the final position of the graph can show you the enthalpy, or the energy in a reaction.
The last thing from the first unit was equilibrium, which is a unit that talks about reversible reactions, or reactions that can go in reverse. Equilibrium occurs when reverse reactions happen the same as forward reactions, meaning that the amount of product and reactant is constant. There wasn’t too much covered here – to put it simply, this part of the unit just required us to know what equilibrium was and how changing factors could affect it.
That was the first… of FOUR UNITS IN REGULAR CHEMISTRY… help…
The second is probably one of the harder ones and bigger ones (a ton of material), and it’s about Thermodynamics. Thermodynamics is probably one of the largest, most complicated, and most vital units in chemistry and even Physics. The first concept we went over is called calorimetry, and it has a lot to do with the heat transfer between objects. In general, when you get two objects that touch each other, (a hot one and a cold one), the hot one has more energy inside of it. The hot one will then transfer energy into the cold one and the cold one will receive that kinetic energy and warm up until they are both evened out at the same temperature. This is called “thermal equilibrium”.
Another part of calorimetry is also figuring out how much energy went into or out of a system. This might sound like it’s a really tough thing to figure out, but once you find that there’s a special equation for it, it quickly becomes easy. This equation is called the “Heat Transfer Equation” and is written with Q=mC * delta T. Q represents the heat that goes in or out of an object and on the other side of the equation are all the factors that affect how much heat goes in. First off is the mass of the object, followed by the Specific Heat (which in short indicates how much energy you need to heat something up by a certain amount) as well as the change in temperature.
TThere’s even more applications with this equation, called the heat of phase change. When you put heat into something, you change its temperature, and when you change temperature to a really big amount, you change the state of matter. Solids occur at the lowest temperature, then liquids when it gets higher, and finally, gases. When a substance changes its state (or phase) the temperature doesn’t change, yet it consumes energy anyway, creating this sort of deal:
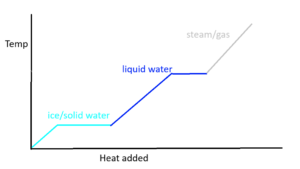
The normal slopes on this graph can calculate the energy using the Heat Transfer equation like usual, but it’s different when the line goes straight, because that’s where the state of matter changes are. Instead, you can use a new equation, Q = m * delta H in order to find the energy put in there. To find the energy transferred over several state changes, all you have to do is find the individual value for each section and phase change, and after that you add all the energy put in together in order to get a grand total.
And then there’s even more – this thing called Hess’s Law which has its own type of crazy problem. A Hess’s problem is formatted kind of like this: You’re given two equations and their enthalpy changes (the change in the energies in the reaction). You have to manipulate and combine them to form a new equation and find that equation’s enthalpy as well. There’s a number of rules of manipulation here: First, if you reverse the order of the equation, you need to reverse the sign of the enthalpy as well. Second, if you muiltiply the equation by any number, you have to multiply the enthalpy as well. You can combine equations and their enthalpies to form new ones, which was the basis of this kind of problem. Weirdly enough, a lot of classes in chem (based off my experience) all learned this a day before the test and it was extra credit for most of them. You’re going to need a decent understanding of algebra before you get here. FINALLY, THERMOCHEM IS OVER…
The gas unit is next, and although there was a lot less material than thermochemistry (I missed a lot in thermochem in this article, I don’t wanna keep it too long haha) it was still incredibly long. You may know the equation PV=nRT and assume the whole unit revolves around just that, but there’s actually quite a bit more to the unit. Let’s backtrack a bit.
Before we formally learned PV=nRT, we learned three more important equations about gases that have some connection to PV=nRT if you look at it in a mathematical sense.
The first of these equations is Boyle’s Law, which states that the volume of a gas is inversely proportional to the gas’s pressure, as shown in the equation psub1*vsub1 = psub1*vsub2. (oh gosh that notation tho) which makes sense as in less space, more particles are crammed together, thus forcing an increased number of particle collisions onto various surfaces.
Secondly is Gay-Lussac’s Law, which states that Pressure is proportional to temperature in the equation Psub1/Tsub1 = Psub2/Tsub2. (In areas with more particle collisions, there is more kinetic energy).
Lastly in Charles’s Law, which states that volume and temperature are proportioned in a V/T=V2/T2 format. (at a lower volume, more kinetic energy occupies the same space)
You might have noticed all three of these equations talk about the same three things: Pressure, Volume, and Temperature. These can be fused together to create the Combined Gas Law equation, which you’ll notice is very similar to the proportions I put in the previous paragraphs about Boyle’s Charles’s and Gay-Lussac’s Laws. The Combined Gas Law is PV/T=P2V2/T2.
The next equation is, at last, the Ideal Gas Law, which is the most well known (I think) out of all the equations in the Gas Unit. The equation is PV=nRT, and you can probably already figure out that P is pressure, V is for Volume, and T is for temperature. But what about n and R?
Well, n is the number of moles in the gas, and it gives you a good idea about how many particles there are and the density inside of the gas. (After doing AP Chem self study during the summer, there actually is a way to apply density to the PV=nRT equation to find molar mass or something, but since we didn’t go over it in regular chem I won’t go into that just yet) And R is some crazy constant, 0.08206 (I have no clue who got it and what alien techniques they had to do to get that number) which apparently helps make your calculations accurate.
There’s one more equation that they talked about a bit but never went too in depth on (I didn’t even know there was an equation for it until I started AP Chem) which is the partial pressure of gases. Let’s say you have multiple gases, each exerting different pressures. The total pressure of all of those gases combined will be all of their separate pressures added together, notated in the equation P sub Total = P subA + P sub B + P sub C and so on until you have all your gases inside. There’s also a million more applications with this, but once again, I’ll save that for AP chemistry.
Our last unit is Solutions, which we didn’t go over much so I’ll be quite fast. They talked about solubility, which is the ability in which a material can be dissolve at, saturated/unsaturated solutions (whether more can dissolve or not) There was also molarity, which is moles over liters…
On top of lectures, we also did a lot of labs. Far more than any other science class I’ve taken in high school so far— sometimes we were working on three lab papers at once. The labs overall were pretty good and you had to integrated whatever we learned from the lectures into a real life situation, which was quite nice. An example of this include a calorimetry lab where we measured the change in temperature of water. A lot of the labs had some pretty dangerous equipment compared to previously. While we were doing some of the labs, we were given a temperature gun to take a look at the temperature of the substances during the experiment. During the calorimetry lab, we put water in a hot plate that reached around 80 degrees Celsius (176 F). Another lab we did brought the temperature of a piece of metal to around 900 degrees F or in some cases even more than a thousand…
So what are my final thoughts on this class? First off, a ton of material (Keep in mind this is just regular high school chemistry, not the advanced placement one that I will be doing next year). I didn’t even cover all of it, and left out a lot of important parts to save you from more confusion. There’s a lot of math, and I would recommend knowing at least some Integrated 2 before you take this, otherwise you might run into a good amount of trouble. And definitely work on it a lot- I spent hours on it and had trouble getting an A.