WHAT THE FUUUUUUUUUUUUUUUU-
How did I survive this?! This is one of the hardest- possibly the hardest class offered at my school altogether! I struggled so much in Regular Chemistry 2- barely made the A range; I got destroyed, honestly, for most the class wasn’t even hard… but now I ace this with flying colors? I genuinely don’t get how this went so well- oh boy, this was a crazy story…
I was paranoid the summer before my sophomore year started, let’s start with that. I was terrified of AP Chemistry- I’d heard horror story after horror story about friends who failed tests- even double digit percentages were hard to get, things along those lines. How could the course be this difficult?! I thought Integrated 2B math’s 10% rate of getting an A was hard enough- at one point the highest score in 2b was just 85%. I didn’t even fully realize it yet, but I was heading into a class where the test averages were just 30%, sometimes even less. This was one of my friend’s descriptions of the class.
Think of being surrounded by armed men with rusty old spears and swords, as well as guns. One of them penetrates your thigh. The other penetrates your skull. For hours at a time, you are repeatedly stabbed with weapons for hours at a time, until a blessed noise rings out, the bell. You drag yourself, bloody and covered with wounds, to another location. This process repeats for 5 days every week – Y.K.
Paranoia moved me quickly. I raced through all the material, trying to get it all in my head before the school year started- I wasn’t going to have any chance of success if I tried to learn it during the school year, because I already think slower than most. I needed to be ahead from the first day until the AP exam came. I studied for hours and hours throughout the first few weeks of summer vacation, finishing each unit as best as I could: Atomic Structure, Bonding, Intermolecular Forces, Reactions and Stoichiometry, Kinetics, Thermochemistry, Equilibrium… and next was Acid-Base.
I hesitated when I reached Acid-Base. I wasn’t sure if I could fully understand it before going over it in class- it was the hardest unit to my knowledge. Could I really survive that? Was I ready? After some consideration, I decided to skip it for the time being – the difficulty of that topic scared me too much, and all the stories I’d heard about it weren’t helping. I moved onto the last unit, Electrochemistry and Thermodynamics.
I slowed down halfway through the summer to focus on other things, a class at a nearby college, and of course, JP2024. I allowed myself to rest- I continued to study extensively, but as new details about the school year and classes came in, I began to change my methods. The units for the first trimester were Stoichiometry, Electrochemistry, Thermochemistry and Thermodynamics, and lastly was Kinetics. Knowing this, I began to change my strategy ever so slightly, focusing more on Stoichiometry now. I became aware of my course roster the day before school started, and my first thought when seeing it was: Who would be a good lab partner?
I already knew one person who I had decided to team up with earlier before the school year even started: A guy named AM – the absolute goat. He was very well prepared for this class, having a strong background in just about every subject in school and a flawless 4.0 GPA so far- he was incredibly smart and I was confident that he’d be someone to work with.
But who else? I needed to choose two more people- looking around the classroom, my options were pretty good, but unfortunately there was a huge problem- most of the good lab partners had already been taken. Everyone had decided to team up with their friends, of course. Most teams looked like they had at least one or two strong people academically, and they were all filled up. Crap, I had missed my chance to grab the best!
Luckily, all hope wasn’t lost. There was only one more table, a table of leftovers, but it did re-spark my hope for a while. There were two of them – a tall caucasian guy that I knew from middle school, and another girl that I’d never seen before. Me and AM, without any other choice, really, decided to sit down at this table and team up with them for the unit. I knew the tall caucasian guy was a really strong person academically- from middle school, I knew he was a very hard worker and enjoyed science. His name was AG. Most of the school had taken Bio-Chem-Chem as freshmen, but he went ahead and began with AP Biology. That was great! We had two strong academic people, three if you count me.
So me, AG, and AM. Who was this last girl?
I don’t know. She identified herself as AJ – I wasn’t sure how good she was academically but I decided that since we already had AM and AG, we could afford to risk one group member who was a little weaker. We all turned our attention to the teacher, who introduced himself to the class.
“There was a robbery in this class last night!” He declared straight away.
Looking around the classroom, there were no visible signs of a break-in. I couldn’t tell if the teacher was serious or joking. “Luckily, we have security footage, ” he added, and then he showed us the weirdest video I’d ever seen.
The video started with my teacher walking into his classroom and heading towards his desk. On his desk, he found a note, which said something like this:
Pay for my lunch tomorrow or you’ll never see Baby Yoda again.
I had a feeling now that this was connected to a lab, but I was still trying to piece together which lab it could be.
On the video, my teacher acted like he was panicking and immediately ran into the hallway in the middle of the building. He went into every teacher’s classroom, went to every teacher’s desk, and stole their black Sharpies.
After seeing this, I realized what was about to happen. “Wait, guys,” I turned to AM, AG, and AJ. “I think I know how this lab works,” But before I could explain it to them, the teacher immediately conducted the experiment in front of our faces, and I missed my two second window to flex on the rest of them, which was disappointing given how hard I’d worked to get ahead in the first place.
This lab was about chromatography and separating mixtures. Did that sound like a whole bunch of gibberish to you? Let me break it down a bit – the general point of this lab was to figure out who wrote the note declaring they had Baby Yoda hostage by figuring out what exactly was inside the ink of their pens- we were going to compare the composition of each marker for the teachers at our school and draw a conclusion on who the culprit was.
To do this, however, we needed to figure out what was actually inside the ink- what dyes was it made of?
The first thing we do is acquire a special type of paper called “chromatography paper” and put a single drop of the ink towards the bottom of the paper, and we label the horizontal position of the drop of ink on the paper.
Next, we suspend the paper in a cup of water and allow the water to travel up the paper. As it travels up, the ink will travel along with it. Because every dye is different, each will interact with the paper in a different way, and so the inks will each separate as they travel along with the water towards the top of the paper.
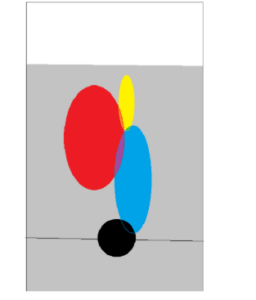
So we had three possible suspects, which were actually just the names of teachers at our school. There was C, L, and D, two of which I’d already had a class from in my freshman year (Biology was from L and Chemistry from C). Without the chromatography being performed on them, all of the ink created by their pens was identical- it was impossible to distinguish one from another, but once we got through the experiment, we’d be golden- it’d be easy to make observations, this was going to be an easy, easy win-
We failed.
Yep, as it turns out, we arranged the experiment like this:
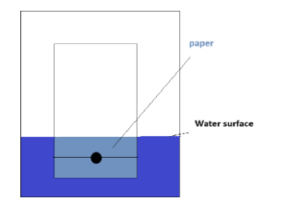
However, this is not the best setup to separate the dyes. Apparently, the water is supposed to be a little lower, arranged like this:
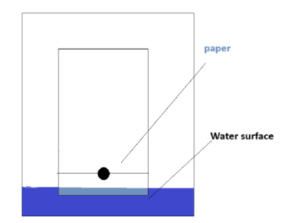
It turns out that this single mistake made all the difference, and that’s why it skewed our results the first day. We had to come into class during office hours the next day to fix our lab, and it turned out it wasn’t only us. Hundreds of people, literally everyone who decided to take AP Chemistry messed up in the exact same way we did, and everyone had to redo it.
Great, only two days in and the entire class already had a serious missing assignment. How could this get any worse? Surely this was as bad as it gets, the next day was already very stressful as it is…
Oh, no, it was about to get a whole lot worse. We ended up finishing the lab and turning it in on time- it turned out that Teacher L was the one who stole Baby Yoda. (Why he did that, I’m not sure. I asked him and he was still in denial) We figured it out because his chromatography sample had a very similar color to that of the sample on the note. Additionally, his chromatography sample’s dyes separated at a very similar level on the paper (It traveled almost the same distance relative to the water as the note’s colors) which made their samples look near identical.
I don’t know who came up with the idea of chromatography, but geez…. Were they trying to make some kind of formless art? Really, these look hella satisfying…



So that was the first part of our class- the wonderful lab about chromatography. As it turned out, this was really just a warmup for the first unit of the class: Stoichiometry. Anyone who takes regular chemistry probably already had a feel for this, and it was probably easier to do most of the skills here than another unit. I’ll skim through it quickly.
The overall unit was made of four smaller modules, each given to us on note sets. We actually print our own note sets because there’s way too much to take in on a regular schedule. Printing it out would end up being stressful for a few kids who typically forgot, but in the long run it usually saved us a load of time because we only had to do annotations for the notes, which weren’t even that plentiful. This might have saved us hours.
Stoichiometry is really just an elaborate sounding way of saying “Unit conversions”. Depending how much algebra you’ve done recently, this could be really easy or really difficult. The first part of the Stoich unit covered the very basics of chemistry – they literally went over the very broad stuff- What is Chemistry? What is matter? What’s the Scientific Method? What about the History of Chemistry? They went over really basic chemical laws, such as the Law of the Conservation of Mass. This law states that the mass going into a reaction is equal to the mass coming out of a reaction. A similar trend can be stated for energy, which implies that the universe always has a constant amount of energy on it.
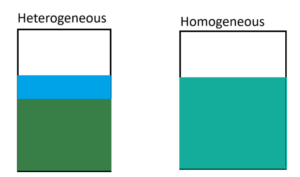
Because Chemistry is so deeply intertwined with math, the teacher also decided to review units such as units of mass (Grams), units for volume (Liters), temperature (Kelvin), and pressure (Atmospheres) and significant figures…. Holy crap, significant figures are annoying. They’re basically a set of rules that defines how many digits your answer can be when you perform a mathematical function onto it, because apparently no regulations with digits doesn’t sit right with most mathematicians. They also discussed precision and accuracy. The note packet ended with a quick summary of how to classify matter with your typical basic terms (solid, liquid, gas, vapor, fluid) but also discussed mixtures of substances as well as the types of mixtures. There’s two types: Homogenous and Heterogenous. The Homogenous type is characterized by how the entire sample visibly appears to be the same across the sample. A heterogeneous sample is a mixture with parts you can distinguish from one another.
The end of the packet usually consists of a dozen or so problems based on the material in the note set that we can use to get some practice on each of them. I’m not going to go through each one, but overall this packet (Chemical Foundations) was not difficult.
The second packet covered Atoms, Molecules, and Ions. It started out by defining several things on the early history of chemistry (Spoiler alert: wasn’t very useful on the test) and described early achievements by people and civilizations. Eventually, they began to discuss some important chemical laws which would form the basis of the rest of the material.
Law of Definite Proportions – The proportion of an element are always exact
Law of Multiple Proportions – If Element A and B form together to create a compound, the proportion of their molecules (Not mass, but we’ll get into that later) will always be reducible to small numbers, such as 2 or 3.
These were the driving forces of our studying of Stoich, and oh… no… it’s gonna get a lot worse soon…
The packet also discovered the history of the atomic structure, which was mostly a review from Chem 1. I’ll briefly run through it here in case you’re curious.

If they’re going to talk about atomic structure, they also need to discuss parts of an atom- there’s quite a lot one could say about an atom’s structure, but all you really need to know is that Protons and Neutrons have mass and they occasionally vary in what’s called isotopes. Electrons exist in different shells around the nucleus. Outer electrons, also called “Valence Electrons”, are known to cause a lot of chemical reactions. Protons typically define what the element of the atom is. Electrons typically define the charge based on bonds, and neutrons… well, they don’t really define anything major. All they do is explain what the hint is.
Of course, Chemistry with a single atom is boring, so we need to discuss how atoms interact with one another and… oh, damn, that’s a lot of words. Seriously, how am I supposed to fit all of this into a single article? Okay, I’m not going to try and bore you with a bunch of words.. I’ll just put this into an infographic because those are more interesting.

Oh, and did I mention the naming conventions that go along with ions and atoms? No? Well, there’s a whole bunch of precedents set by old men that I barely remember myself. There’s several different types of molecules beyond what I already mentioned, and each of them has their own system of naming. So you don’t just have to memorize the systems of naming, you have to memorize a system for using each of the names.
There’s a system of naming for:
- Type I – Metals and Nonmetal Ionic Bonding (Alkali)
- Type II – Same thing as before but with a different kind of Metal (Transition)
- Type III – Covalent compounds (Between nonmetals)
- Acids
And yeah, that’s only half the unit’s material skimmed over so far! This class is so fast paced that nobody can keep up- the teachers usually lecture through this thing in a really short amount of time, so I can’t really absorb any of the material.
And the next note set is what nobody was waiting for… Stoich.
Oh, boy, here we go…
There’s a LOT of calculations you need to know how to do in AP Chemistry, but I think Stoichiometry is a really good unit for you to know just how much you need. There’s the average atomic mass, which you can use to figure out how much of a sample is a certain mass as well as the percent abundance, which is the percentage of an atom’s element in a sample.
And finally, the mole. The mole is a unit of essentially how many molecules are inside something, and it’s one of those concepts where you learn it and it sticks with you for the rest of your learning career. A mole is defined in a reaction as the coefficients, so the little numbers in front of the elemental and compound symbols. They can be converted to grams really easily using the molar mass, and if you’re wondering what a molar mass is, you can easily find it on this part of an element’s box in the periodic table.
Is there a way more complicated version of explaining this? Yes. But this is all you really need to know in the class. You can repeat this process for any element to use in calculations. Remember, this 6.94 grams per mole is a ratio, so you can actually solve for moles or grams using dimensional analysis.
Guess what? It turns out… that was just one unit conversion that you can do with moles- the entire unit is really just about what you can do with moles, and there is a lot more down the road. A mole is also defined as a very specific number – sort of like how humans associate a dozen with the number twelve. The only difference is, one mole of something is equivalent to having 602 sextillilion of something. I’m going to rewrite this as 6.022*1023 since that’s simpler, but that’s the number of atoms in a mole.
There’s even more ways to solve for moles. There’s another equation you can take based on concentration and volume, M = moles / Liters, which should be easy to solve if you use algebra correctly. It’s very useful, especially if you’re studying solutions. You can also solve through this equation PV= nRT, where n is the number of gas moles and the other values include temperature, pressure, volume, and some random constant that I couldn’t derive even if my life depended on it. Lastly, there’s an equation that you can use to solve for gas moles at specific temperatures, where 22.41 Liters of gas gives you the same number of moles every time.
So what do you even do with these dimensional analysis skills? As it turns out, there’s many things you can make.
-
- Molecular Formula – The elemental symbol that defines most chemicals. You know the H2O “water” formula you were taught back in 3rd grade? That’s a molecular formula.
- Empirical Formula – Sometimes you’ll encounter a molecular formula like C2H4 or H2O2 where the subscripts on each element can be simplified. This is called the Empirical Formula. An example of this could be how the empirical formula of H2O2 is HO, because that’s how simplified the numbers can get. One of the most common problem types requires you to solve for the empirical formula first, then try and figure out the molecular formula afterwards.
- Percent Composition – All you need to do here is find the total mass of a certain element or compound, then the mass of a mixture. Divide the element by the mixture, and you get the Percent Composition.
- Hydrates – A super complicated process where you have to figure out the ratio of a solid to water when the solid is inside the water.
- The one strategy that doesn’t have a name but is super important – Another important use of mole conversions is using it in a reaction and figuring out how much product is produced based on what goes in and what comes out. It’s probably the most straightforward part of stoichiometry, once you understand it.
- Limiting Reactants – Sometimes they’ll make it a little more difficult and ask you find a limiting reactant. Basically, they’ll give you two masses for two reactants in a reaction, and you have to figure out which one will run out first. There’s several ways to do this, but the most efficient way that AP Chemistry students prefer is to figure out how much Reactant B can react with the given amount of Reactant A. A basic inequality can deduce what the reaction’s limiting reactant is.
- Percent Yield – Kind of like the Percent Composition but you’re comparing products produced in theory compared to what’s actually given in an experiment.
With all this knowledge, you can pretty much do any stoichiometry problem. Just remember these last few extra tips.
- Some elements are diatomic (Hydrogen, Oxygen, Fluorine, Nitrogen, Chlorine, Bromine, Iodine)
- Balance equations too, otherwise your coefficients will be wrong.
You’ve probably noticed that I threw around the word reaction a lot. Most of the stuff above can be applied to reactions. As you’re probably aware (or you saw this coming) there’s eight different reaction types that need to be known for AP Chemistry. Some are more complex than others.

Those five are probably the most basic reaction types. The other three are called precipitation, Acid-Base, and Redox Reactions. Luckily, for Stoichiometry, all you need to know is the Precipitation Reaction (Because any more material and I’d die)
You know how when you put salt into water it dissolves and becomes a solution of salt water? That development occurs through these things called intermolecular forces and polarity. Essentially, the water has a slight positive and negative charge which pulls the salt completely apart. However, water is a very finite thing on our planet (we don’t have water shortages for nothing) and eventually there will be a limit to how much salt it can dissolve. Once it reaches its limit, any salt added afterwards will just sink to the bottom and form a solid, which is called a precipitate. Having more salt than the water can hold is called supersaturation.
Not every salt becomes supersaturated. Some salts don’t even dissolve in water even if the water has a capacity for it- this really depends on what the salt is made of. You’ll need to memorize several rules to figure out what does and doesn’t form a precipitate.
Wow, that was a lot of material for one unit- I genuinely tried to simplify this thing as much as I could, but there’s still a lot I had to leave out for the sake of time. The main problem isn’t that I don’t know how to do any of these skills- it’s that there’s so many to choose from that often times I don’t even know how to do it in the first place. I’ll be given some information to start, but it’ll typically be super random- I won’t have any clue how to find it, so…
Just be ready for that.
Each unit of the class consists of a bigger and a smaller lab. The smaller lab in our case was the chromatography lab I explained earlier. The bigger lab ended up being based on a little thing known as hydration, which uses real data in a chemical reaction to find the unknown identity of a chemical.
AJ and AG ended up doing absolutely nothing on it.
And oh boy, AM was so pissed. AG was supposed to be the smart, hardworking one who did his stuff well! And this AJ girl- whoever she was, had to do her job too! At least I contributed, but AM was angry and he ended up lashing out at both of them the day he got the chance. AG and AM needed to contribute to the next lab, no matter what.
And with that out of the way, let’s discuss how absolutely destroyed everyone was about to get on the test.
Because AP Chemistry is so difficult, the teachers decided that a few days before the test we should get some time to do some real authentic practice problems to get a feel for what the test would be like. They gave us what they liked to call a “medium difficulty problem”. It wasn’t too hard, my teacher told me, but it wasn’t going to be the easy problem either.
We all got our pencils ready and prepared to turn the paper over the second we were given a chance to. “Aaaaaand…” My teacher said quietly, before yelling out, “ENJOY!” And we all turned our papers to the other side and started the practice test.
So anyone with a decent background in chemistry and mathematics might go ahead and see what I’m writing here for the material and say, “Oh, this isn’t it so bad.” I completely agree with you. The only thing we’re doing here is just dividing and multiplying and adding numbers- it’s just fourth grade math! How could anyone struggle with this? It’s an easy test! I knew all the stuff I needed to, and I was completely ready for it.
The practice test had four questions. All of these questions were doable with the skills we’d learned, and I’d learned them well. The first question asked what the empirical formula of the gas was.
That was not bad, just use the given mass percentages on the gas to find the empirical formula. First question was done with ease.
But oh boy, the rest of the test was about to be an absolute hellhole for me.
Question two: What fraction of the fluorine of the original compound is in the solid and what fraction is in the gas after the reaction?
Um, what? That question was asking for a lot of things. What does that even mean? How do you do that? I tried to think, and I came up with a process using the skills I knew that I thought would solve it, but I really wasn’t sure at all. I thought and thought and thought… what was this question? How do you even do it?
I ended up writing something for the second answer, but it wasn’t a complete answer, and it certainly wasn’t anywhere close to being right, but it was something. Hopefully if I got to a situation like this later on on the actual test I’d be able to do something similar there and get a few points back, but I wasn’t entirely sure how secure my spot was going to be. For now I continued onto the third question which was asking again for another empirical formula- that was an easy one! I could do that with no problem- it was entirely doable and I was really consistent when I was trying to find one- NOPE. Unfortunately for me, the entire practice test actually counted as just a single problem, which meant every part of it was built off the last. The most I could do was try to come up with something from the first part, but there wasn’t enough information for me to get a complete answer in part C either because I never finished part B. Great.
And then the last part, Part D, was also really easy but like part C, it was also built off the previous problems that I never bothered to start. I ended up coming up with another half answer. I tried to think of other ways to get past all of the roadblocks I’d encountered, which there were quite a lot, but I genuinely had no idea what I could be getting. I’d pretty much done everything I could think to do.
I ended up sitting there like an idiot with no idea what to do for the rest of the time on the practice test.
When the time ended, I quickly realized that I wasn’t the only person who failed utterly. I could see AJ had also put random filler answers for all the questions like I did. AG was also extremely confused and only answered a small part of the overall questions. AM was the only one who had a complete answer for all the questions, and even then he didn’t look confident. I took a quick glance around the room to see how everyone else felt about the practice test, and nobody looked any more confident than I did.
“Alright!” My teacher called from the front. “How many of you feel like that test went really well?”
Not a single person raised their hand. I was quite surprised to see this, because our class was full of smart kids, but either this test had killed everyone or those who were confident were simply too scared to be the only ones raising their hands.
“And how many of you feel like you bombed it?” The teacher asked.
At that exact moment, every hand in the class went up. Everyone immediately started chattering in surprise, but the teacher didn’t seem disappointed at all despite the fact that most kids only answered the first question and failed the rest. “This is completely normal,” The teacher promised. “Everyone gets a score like this when they first start. It’s knowing what to do that’s the hard part. Once you know what to do it becomes easy.”
Going over the answers, my teacher’s description of these tests were completely right. I could do all of these problems with no issue- it was just that they were all so weird and tricky and I didn’t know how to do any of them because I was unfamiliar with all of their unique formats. It was doable, though, and I could finish it without any issue if I was able to figure out the procedure immediately from the beginning. I could do this. I could do this.
A week passed by, and we studied more and more and more. I had friends come over to discuss content that we were confused on and I also did as many practice tests as I could get my hands on. I failed every single one of them, but as time went on I was failing less and less spectacularly. I heard from friends that the test was going to be out of about 28. If I focused hard, maybe I could get at least a 21… a 75% wouldn’t be so bad.
At long last, the day of the test came. The first part of it was a multiple choice. As we entered the room and got our materials and calculator, my teacher prepared us with a pep talk and a calculator check. “Enjoy,” He declared, and we started the multiple choice.
So the problems I knew how to do from the start, which was great.
So why was I panicking?
Each problem took about a million years to solve. I punched numbers in my calculator, scribbled down the calculation setups on my scratch paper as quickly as I could, and I started sweating, which only made me work harder and faster, but these tests were just too long, and there wasn’t even close to enough time. I had to finish this? HOW? By the time I reached the 10th question out of 30, I could barely work. I was dying in frustration because I couldn’t do any of the problems quickly. How was I supposed to even finish the test, let alone ace it?
I kept myself going through the test – I had to do things quickly, but I tried my best to get it done…
“TIME!!!!!!!! On the collar!” Shouted my teacher to signal that the test was over.
I wasn’t ready for him to yell that loudly, so I immediately started shaking when I heard it- I was shaking for about a minute straight before I regained composure and turned in my test. I didn’t even finish everything- I still had about three questions that I didn’t have time to look over, so I just picked a random letter choice and hoped I’d get at least one of them right. That was a terrible test, and nobody felt good about it. It turns out that skipping three questions like I did was already a better situation than most people. Almost everyone I talked to skipped at least 8 or 9 questions, some as many as 15. It wasn’t even over yet – infamous Free response was next, and it was coming the next day.
The free response in a chemistry test at our school usually has four parts, each part with its own smaller questions that it asks to rob us of our time. The first two parts are usually the longer ones. The first part is typically a really difficult problem that takes a really long time to solve. The second one also takes a while, but it’s generally easier since it is always centered around the lab (in our case, hydration). The third and fourth questions are typically shorter and don’t take as long, and at times they are centered around a different lab.
When we started the test, the problem…
What the hell…
They threw about a million pieces of information at me, but it was all of the information that I didn’t know how to use. I’d never seen a problem like this and wasn’t sure how to make a procedure on the fly, but I had to at least put in some effort. The overall problem had 6 parts, and after burning through almost ten minutes, I had only finished the first question of the first part of the test. Great. That was almost a quarter of my time gone, and I’d done two percent of the work I needed to do.
I kept looking at the first question, but the second part of the first question went completely over my head, so I decided to move on to Question 2, which was the easiest question on the test by a long shot since it was about the lab. I already knew the procedure from the lab so it all made complete sense. I just had to do the exact same thing, only with different given numbers. I answered everything in only five minutes, despite Question 2 being the longest.
Question 3 was the first short question, and I was extremely confident in this one- I knew exactly how to do it, and all the procedures was easy. Similar story with Question 4.
Compared to the Multiple Choice, everyone felt a lot better about it than before. The FRQ was so good one of my friends wanted to marry it- it was entirely possible to get an A, we were sure. It came to light that the exam was out of 28 points. Questions 1 and 2 were each worth 10, and 3 and 4 each worth 4.
And then the day came a few weeks later to grade our Free responses (or FRQs). Peer grading was the worst. I’d forgotten all my answers to the problems somehow and was terrified when I didn’t recognize anything. The person who I graded was absolutely failing the test, and I was worried I’d get the same fate. The highest grade anyone in my table peer graded was a thirteen, and that was certainly saying something about the standards of the test. I couldn’t even expect to get a 10/28. When the time came to look at our scores, I predicted that the most I’d be able to get was a 5/28, which didn’t seem so bad now that the person I graded only got a 4.
I received my paper and…
14/28. 50%. Fail. Actually, a little better: the person who graded me actually made a mistake, so it was a 16.
I’d probably break down in tears if this was a test in a normal class, but not this time. A 50% was a great score here. I’d done it. This was one of the hardest tests in the entire class, and I got an A. Yes!!! Hope started to return that I might be able to get an A in the class after all… I’d made it through the first unit and gotten a 93%, after all! But oh boy, things were about to get a lot worse…