The 3rd Class I did in the 2nd trimester of my freshman year was a chemistry class. Once again, I’m doing Bio-Chem-Chem. If you forgot what that is, here are the science class paths:
Bio-Bio: The generic science path that some people do. It’s supposedly the path for those who might not be interested in science.
Bio-Chem-Chem: The “advanced” science path which allows you to get more science in. People usually take this one in order to rush towards the AP science classes in their sophomore years.
AP Bio or AP Chem in Freshman: This is the real rushing path. Not too many people do this, so if you’re willing to study some biology or chemistry in the summer…
Now let’s talk about the curriculum. Chemistry, as hard as it sounded, being the “advanced” path, might actually be easier in Bio-Bio (My opinion after taking it)(Modified opinion: NEVERMIND. CHEM 2 IS KILLING ME HELP PLEASE GET ME OUT OF HERE okay it’s not that bad but it’s certainly harder than whatever bio-bio could ever be. Anyway, back to the article) . It has a lot of math involved, only far simpler than that of the Integrated 2b class I did at the same time. There was a lot of memorization in Chem, but the procedural memorization processes of getting answers allowed me to get most concepts quicker than biology.
The first unit mostly focused on atoms and nuclei: we started with lectures on the history of atomic theory (which covered all of the important models of the atom that scientists made throughout the centuries) before then moving on to talk about the subatomic particles (protons, electrons, and neutrons), and then after that we started covering isotopes, which are types of atoms that have the same element.
After covering most of the basic structure of the element, we started to place an emphasis on the nucleus. Most isotopes of certain elements are unstable, and when that happens, their nuclei will undergo change in order to regain their stability. We spent a few days studying the types of change that will happen in order to get stability, collectively referred to as types of nuclear decay. The amount of time that it takes for the decay to happen depends on a substance’s half life, which is the time it takes for half the substance to decay.
The next part of the course focused on things that happen within the atom, as well as start on the periodic table. We talked about what it actually means to have a place in the periodic table- or in other words, we discussed the different trends that you would find depending on what direction you moved in the periodic table. These trends included some very important properties which include electronegativity, or the ability of an atom’s nucleus to pull electrons towards it or the atomic radius, which is pretty self explanatory: just the radius of the atom. These two in particular had very easy to follow trends. An atom’s electronegativity will typically increase as you go right and up, but the furthest right column won’t have any electronegativity since they don’t need to pull electrons (They have a full octet shell).
I hope that all made some sense. Three months worth of material is a lot to take in in a few short minutes of reading.
Going on now- we start on the unit of bonding. You might be familiar with some of this material, especially if you’ve studied chemistry in the past. Bio-bio kids might even know this too because a lot of biology includes chemistry in topics like organic chemistry as well as the reactions of enzymes and photosynthesis. There are two types of bonding: Ionic and Covalent.
Wait, but there’s also metallic bonding too!
Yeah, that is true, but the course only covered these two major types of bonding. The Ionic type of bonding occurs when an atom, typically a metal, gives an electron to another atom, a nonmetal. Covalent bonds usually happen when two atoms, both are nonmetal, share electrons. The number of electrons they share and the atoms that do this really depend on how many electrons each atom has. Some atoms can also create compounds by simply bonding with another atom of their same element. These are called diatomic molecules, and they happen to only a specific few elements. A few of them include Chlorine, Fluorine, Hydrogen, and Nitrogen.
On top of that, we learned how to actually name compounds that were formed when elements bonded. They were very similar and basic formulas, such as (NaCl) is Sodium Chloride. In general, naming them isn’t too hard if you know the formula. It creates some weird sounding words at times, like the word “Hydride”, but the formula has no exceptions to my knowledge. Don’t worry if it sounds weird!
We also talked about a little thing called polarity in compounds. Never heard of it? In short, polarity occurs when part of a molecule has a positive charge and the other has a negative charge. This happens because electrons, which carry a negative charge, will move closer to an atom if it has a higher electronegativity. This makes one side of the molecule have a positive charge, while the other has a slight negative charge. Depending on the differences in electronegativity, a bond can be either polar or nonpolar covalent. The higher the difference, the higher the charge, and the higher the charge, the more polar it is. Eventually, the difference number will be so high that the bond can’t even be polar anymore, and that’s ionic. Because one electron fully leaves the orbit of its original nucleus in this bond, the difference is huge. The different positive and negative charges in atoms can attract and repel each other as ions, but the same is also true of polar covalent compounds. They all have slight charges and therefore interact with each other with a little something called Intermolecular Forces, or IMFs if you don’t have much time, which are defined as the attractions between molecules.
The first type is called a Dipole-dipole, and it works in a very interesting way. Imagine it like this – you have these two molecules, each with a Fluorine and a Hydrogen atom. The two of them have a big electronegativity difference, and therefore the molecules are polar covalent. The Fluorine has the higher electronegativity, so it pulls the electron closer to it and gains a very slight negative charge in the process.

Now imagine that these molecules, having the same orientation, get very close together. What do you think would happen?
Well, since chemistry and physics is generally based upon the concept of “opposites attract” for whatever reason, we can see that the Fluorine and Hydrogen would attract each other. This creates the “dipole-dipole”, which looks like this:
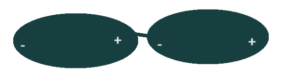
This particular example can also be called a Hydrogen Bond, which occurs when Hydrogen atoms participate in Dipole Dipole events with Nitrogen, Fluorine, or Oxygen.
But what about when the molecules are nonpolar or noble gas? They’ve got no charge, surely nothing can happen to them…
Apparently IMFs still do affect atoms and molecules that are nonpolar or noble gas! It’s just not a Dipole Dipole or a Hydrogen Bond. Here’s an example: Consider a normal noble gas atom with a positive nucleus and electrons around it. Then imagine a molecule which is polar coming at close proximity to the noble gas atom with its positive side pointed towards the electrons. What happens?

The electrons in the noble gas atom all move closer to the positive charge, of course! Because of this, a brief positive and negative charge are created in which the noble gas atom technically becomes a dipole for a short moment.
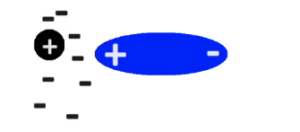
Through this, we had to learn which molecules were polar and how they might react if they came closer with one another- this was by far the hardest unit. After this was the final review, and the class was over!
How did we actually absorb all this material? Most of it was lecture based. Every week, he (our teacher) created a slideshow filled with all the material that we would be learning for the week. When it came lecture time, our teacher would put up the lecture slides and start talking about whatever the lecture was about. Everyone else would take notes, which were relatively easy to follow and I was able to keep up with.
A lot of the material we learned could also be further solidified with understanding from the numerous labs we did. We did quite a few labs this tri, more than Bio 1 for sure, but it wasn’t a huge amount. I’d say we were getting one about every two weeks, and each time we did they were usually quick and easy to get going and to finish. We occasionally also played Kahoots to get the material in further.
The test format was also simple, typically having a muiltiple choice and a free response section which we would do on different days. So, yes, about ten days of the entire class were just spent taking tests the entire time. What are my thoughts on this class overall?
You’re likely going to have to take this eventually, regardless of when. I’d take it later on if I wasn’t interested in doing bio or chem or anything related to that. In short, it really depends on your interests. If you do want to do it but are afraid of the difficulty it might bring with taking the harder path, I can assure you that it’s not amazingly difficult.